

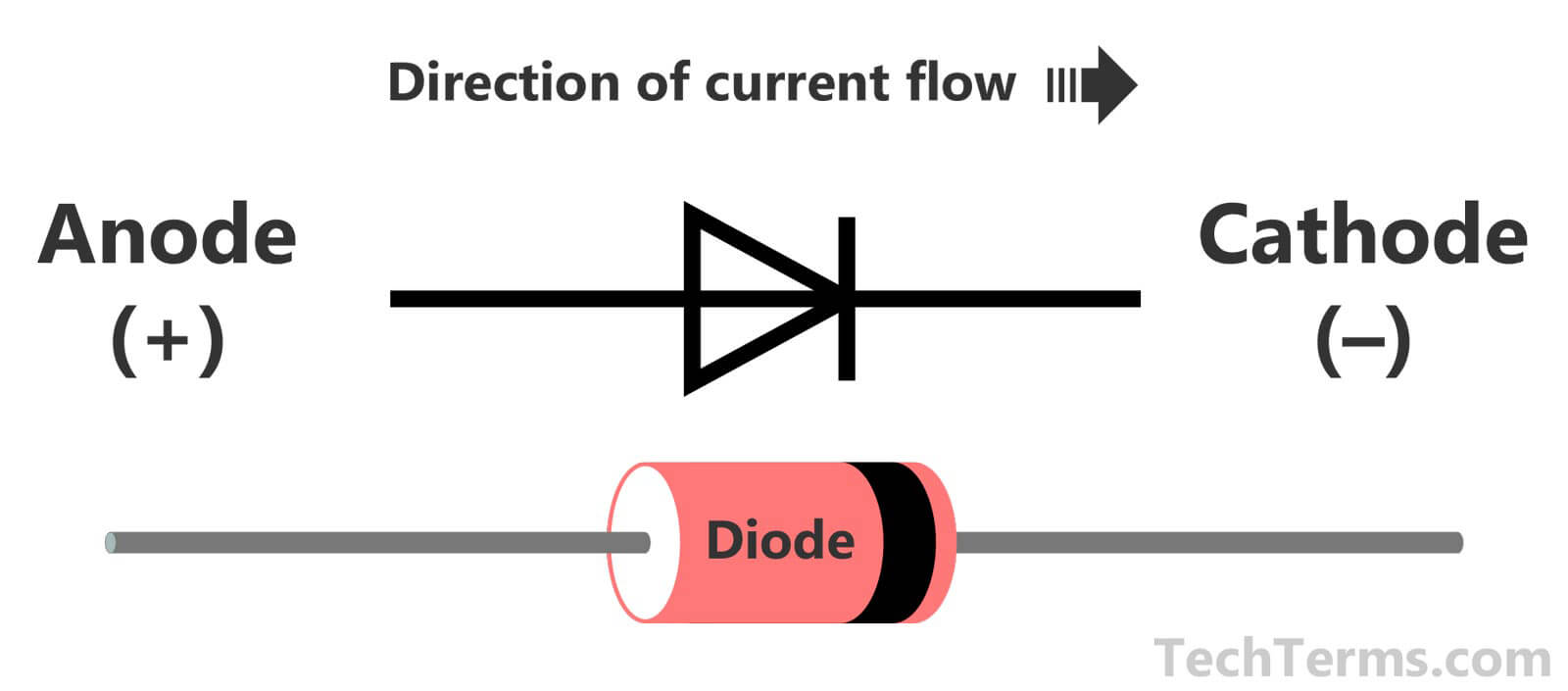
The Cathode is the positive or oxidizing electrode that takes electrons from the external circuit and reduces them during the electrochemical reaction.Īnode attracts negative charge (repels positive charges)Ĭathode attracts positive charge (repels negative charges)Īnode referred as the source of positive chargeĬathode referred as the source of negative charge

The anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during an electrochemical reaction. Representation of Cathode and Anode in batteriesĬells are made up of three essential components. Vacuum tube - In vacuum devices such as cathode ray tubes, the anode is always positively charged and collects the electrons released by the cathode through electric attraction.The anode supply holes connect with electrons from the N-doped area, resulting in a depleted region. Anode diode - The P-doped portion of a semiconductor diode corresponds to the anode and brings holes into the joint area.Electrolytic anode - The anode is the site of oxidation in electrochemistry and contains the positive charge in contact with the electrolytic cell.Galvanic cell anode - A negatively charged electrode from which electrons travel out through the circuit’s outer portion.The battery will die once the anode has completely eroded (or lost charge).Īnodes are classified into several types: The conductor (whether a metal wire or a tube) is how we access the electricity produced by the anode and, ultimately, how a battery powers our devices. It floats in an electrolyte solvent and slowly deteriorates as electrons flow along a conductor to the cathode. ACID, which stands for “anode: current into the device,” is a popular mnemonic for defining an anode.Īn anode is a metal that oxidizes, such as zinc or lithium, and thus loses electrons. Typically, the direction of the current is always the inverse of the flow of electrons.Īs an example, electrons travel from a positive charge, or anode, into an electrical circuit. Indirectly heated cathode: The filament is not the cathode in this type of cathode rather, it heats the cathode, which subsequently produces electrons.Īn Anode is a highly polarised electrode through which electric current flows into a device. In a nutshell, this is how a battery produces electricity.Ĭopper oxide, Lithium oxide, and Graphic oxide.ĭirectly heated cathode: In this case, the filament serves as the cathode and directly emits electrons. Both the anode and cathode are immersed in an electrolyte solution, and electricity flows from the negative to positive parts of your battery via the conductor. As a result, the movement of electrons is diametrically opposed to the flow of current.Ī cathode is essentially there to receive electrons from the anode. Electrons are electrically charged negatively. A conventional current indicates the direction of positive charge movement. The mnemonic Cathode Current Departs can help you remember this.
/how-to-define-anode-and-cathode-606452_FINAL-0fb3b21b79564acd9ef7c729a2bcd98e.png)
It is an electrode from which a polarised electrical device’s current flows. Electrons enter the cell through the cathode, where they undergo a reduction process.Įlectrodes are classified into two categories. The anode is an electrode where electrons leave the cell and oxidation occurs. This is also known as an anode or cathode in an electrochemical cell. If the substance to be transformed is an electrode, the reaction is frequently one in which the electrode dissolves by releasing electrons.Īn electrode is an electrical conductor that makes contact with a circuit’s non-metallic circuit parts, such as an electrolyte, semiconductor, or vacuum. Components of the solution also travel to the opposite electrode (anode), where they give up their electrons and are transformed (oxidized) to neutral elements or new molecules.


 0 kommentar(er)
0 kommentar(er)
